Laboratory of Synaptic Proteostasis
The overarching goal of our research is to determine how protein mishandling contributes to synaptic dysfunction and aging.
The role of impaired synaptic vesicle machinery proteostasis in Alzheimer’s disease pathogenesis. Synapse loss represents an important and early feature of Alzheimer’s disease (AD) that correlates with the severity of dementia. The mechanisms leading to synaptic dysfunction and loss are not fully understood, but both direct and indirect effects of Abeta peptides and Tau pathology are recognized as key drivers. Our team has recently discovered that there is impaired protein degradation selectively in axon terminals that results in elevated abundance of synaptic vesicle associated proteins in early stages of AD pathology. Intriguingly, we observe a relationship between these observations and the effects of the atypical antiepileptic drug levetiracetam that is currently the subject of several Phase II clinical trials for AD. Thus, we have uncovered a potential mechanism that may explain the therapeutic benefits of levetiracetam as well as targets for future therapeutic intervention.
Enhancing cochlear proteome fidelity to prevent noise-induced hearing loss.
The goal of this project is to develop a novel, targeted therapeutic to prevent noise induced hearing loss. The proposed research strategy is based on our finding that noise exposures causing synapse loss, severely impair the cochlear proteome and active the proteostasis network. Our recent results revealed that acoustic trauma activates and overwhelms the ubiquitin proteasome system in spiral ganglion neurons. Taken together with the previous finding that loud noise activates the heat shock response, it is increasingly clear that altered protein quality control plays a central role in the cochlea’s response to auditory insults. The rational is that small molecule regulators of the proteostasis network can protect the cochlear proteome and prevent synapse loss through the activation of stress-responsive signaling.
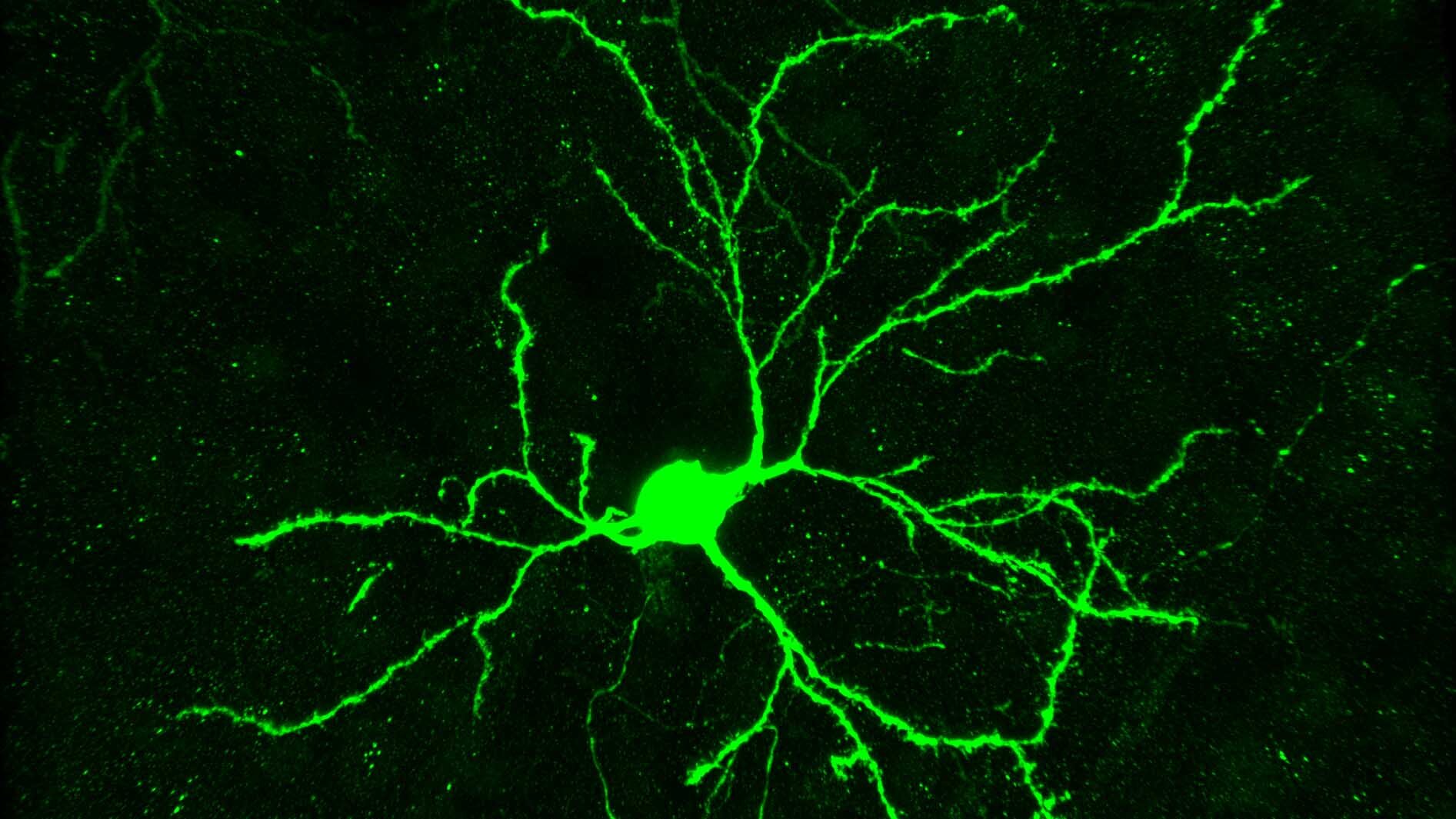
Neuron-specific protein expression at hippocampal and striatal excitatory synapses.
Combinatorial expression of postsynaptic proteins underlies synapse diversity within and between neuron types. Thus, characterization of neuron-type-specific proteomes is key to obtaining a deep understanding of discrete synaptic properties and how selective dysfunction manifests in synaptopathies. To overcome the limitations associated with bulk measures of synaptic protein abundance, we developed a biotin proximity protein tagging probe to characterize neuron-type-specific postsynaptic proteomes in vivo. We currently applying these new tools to advance our understanding of synaptic dysfunction in autism spectrum disorders.
The role of long-lived proteins in development and aging. The goal of this project is to accelerate our understanding of the biological relevance of long-lived proteins (LLPs), which have lifetimes measured in months and, in some cases, are as old as the organism itself. LLPs can be both detrimental and vital to cell and organismal survival. For example, LLPs such as nucleoporins, ATP synthase, and histones have been identified as vital components of large intracellular structures that play key roles in providing long-term structural stability essential for cellular homeostasis. Yet, LLPs are likely to accumulate damage because of their extended lifetimes and thus create vulnerability to underlie disease. Previous studies have been informative but only provide a snapshot of protein lifetimes. The objective of this project is to use LLPs as probes to uncover new and unexpected aspects of developmental biology, points of vulnerability for aging, and pathway beacons of impaired proteostasis in neurodegeneration.